Our current projects work toward our research lab’s main goal of understanding the relationships within the gut-brain axis during pregnancy and postpartum. We strive to gain knowledge that could eventually lead to better prevention and more accurate diagnosing of perinatal mental health problems and spontaneous preterm birth.
Perinatal Mood Disorders Heading link
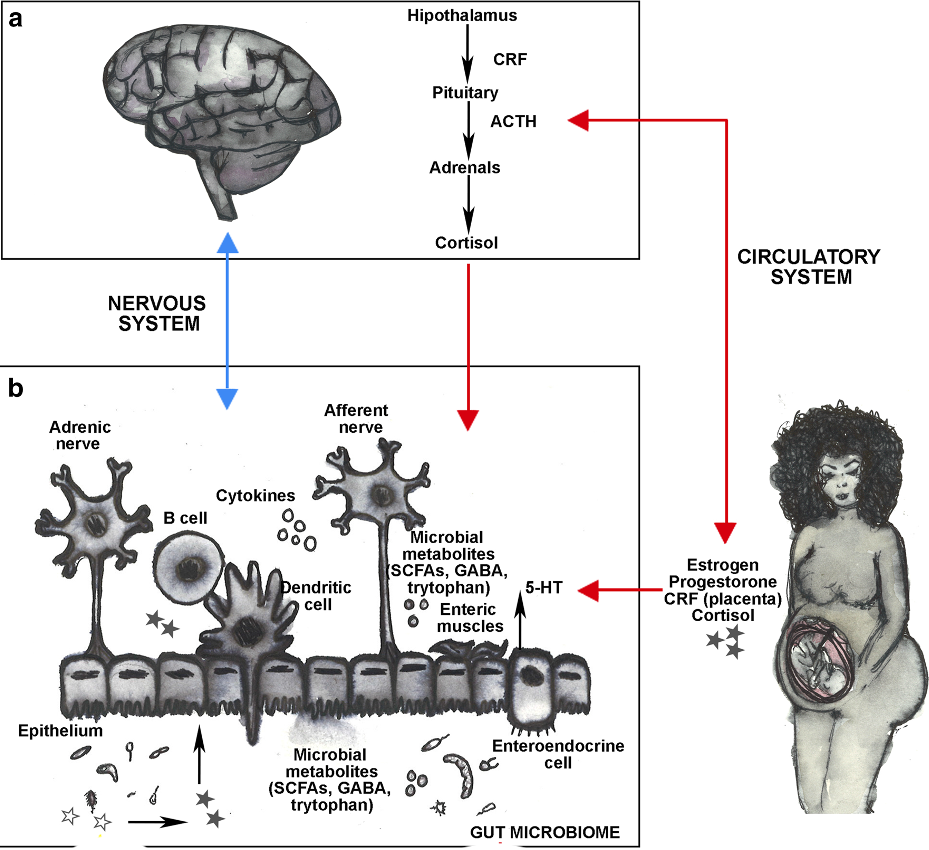
It is estimated that approximately 10-20% of women experience depression during pregnancy and postpartum. Untreated depression during pregnancy increases the risk of preterm birth, low birth weight, and intrauterine growth restriction, all of which have life-long consequences for infants later in their lives. Our current knowledge of the pathobiology of depression during pregnancy is limited by an incomplete understanding of the biological mechanisms contributing to it.
The gut microbiota (i.e., bacteria, archaea, fungi, protists and viruses) and mental health are linked through the gut-brain axis, which mediates the complex, bidirectional interactions between intestinal bacterial communities and neurological, immunological and endocrinological processes. The microbiome can be manipulated (e.g., antibiotics, prebiotics, probiotics, diet and exercise) making it a useful target for the development of new therapeutics that women may perceived more natural and thus increase the intake and adherence of medication to treat mood disorders in expecting mothers. Our group is exploring the complex and dynamic interactions between depression during pregnancy, the maternal microbiome and the maternal neurological, immunological and endocrinal systems using experimental and computational Systems Biology approaches.
Ovarian Follicle Development Heading link
Tremendous advances have been made in the last 40 years to understand mammalian reproductive biology and in vitro fertilization (IVF) is one of the most remarkable outcomes. Reliably producing a competent female germ cell—an oocyte—that can be fertilized entails a deeper comprehension of ovarian follicle maturation, which is a very complex process that includes meiotic maturation of the female gamete, the oocyte, together with the mitotic divisions of the hormone-producing somatic cells. During ovarian follicle maturation, a primordial follicle that is composed from a handful of cells, has to grow into an antral follicle, 10 times larger, in order to attain a competent oocyte.
Ovarian follicle maturation requires highly orchestrated paracrine, autocrine, endocrine and juxtacrine signaling that has to occur between the oocyte and the supporting somatic cells, granulosa and theca cells. Additionally, ovarian follicle development is not possible without the adequate supply of nutrients to the oocyte, as the oocyte must produce and store all the components required to support multiple divisions after fertilization. Moreover, ovarian follicle maturation metabolism and signaling are largely unknown, especially at the early stages of follicle development. Therefore, we are developing intra-cellular and inter-cellular signaling networks and context-specific metabolic models during ovarian follicle maturation in vivo to identify possible growth factors and metabolites to add to the culture media to grow human primordial follicles into mature oocytes in vitro.